The bioconversion of natural gas into liquid fuel has attracted much attention as a promising approach in recent years. However, the selective hydroxylation of methane-the main component of natural gas-has been one of the major challenges for the scientific community.
Researchers at the Qingdao Institute of Bioenergy and Bioprocesses Technology (QIBEBT) of the Chinese Academy of Sciences have made headway towards more sustainable and economic fuel production by developing a biochemical approach to allow more control over the conversion of natural gas into potable liquid fuel. The study was published in ACS Catalysis.
Methane and propane, another component of natural gas, are organic molecules called alkanes. Consisting solely of carbon and hydrogen atoms, alkanes need to be significantly processed before they can be used in fuel. The process includes introducing oxygen and hydrogen, called hydroxyl groups, into the alkane. The atoms rearrange themselves, producing an alcohol that can be used as fuel.
The process is indirect due to how selective alkanes are when reacting to the hydroxyl catalysts. Researchers have worked on engineering an enzyme that would uniformly speed along the small alkanes reaction to hydroxyl groups needed to produce fuel. This has been a long-standing issue because of the inability to directly hydroxylate small alkanes. With current processing, some alkanes are too reactive and renders the resulting fuel useless.
To control which alkanes react and to what degree, Prof. CONG Zhiqi from the QIBEBT and his team focused on several protein variants of P450 monooxygenase which help the process of introducing hydroxyl groups into alkane molecules.
There are more than 41,000 variants of the enzyme, all of which can cause different levels of reaction.
They achieved controllable selective hydroxylation of propane through what Prof. CONG calls an artificial P450 system driven by hydrogen peroxide. The system consists of a dual-function small molecule (DFSM), hydrogen peroxide and variants of an engineered P450 enzyme called P450BM3. The engineered P45BM3 is primed to react to the hydrogen peroxide, and the DFSM holds the enzyme and hydrogen peroxide together, allowing the reaction to occur.
The reaction continues over to the propane, successfully converting the alkanes into alcohols that can be turned into fuel. The researchers found that the system has comparable or better catalytic properties than the only known peroxide-dependent natural enzyme of small alkanes, depending on which variant of P450BM3 they used.
In engineering the variants, they replaced the substrates on the part of the enzyme that bonds with the hydrogen peroxide with more reactive versions, which helped otherwise inert carbon bonds break apart and bond with other available atoms.
"This study gave the first example of direct small alkane hydroxylation by the peroxide-driven P450BM3 variants. This substantially expands the synthetic toolbox toward the development of a practical catalyst for fuel processing. We hope we can further tune the enzyme for use in methane oxidation, as well," Prof. CONG said.
The researchers are now researching the specific molecular mechanisms of the reactions, and plan to use that information to develop similar systems for use with other natural gas components, such as methane.
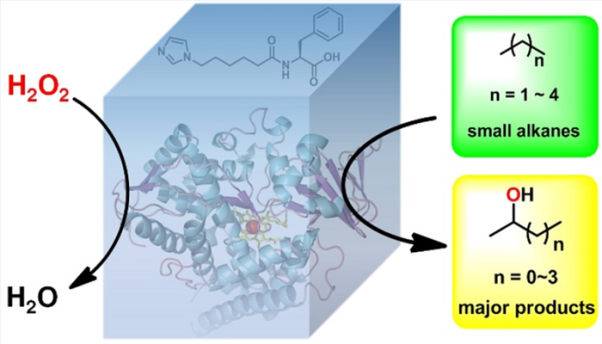
Engineering an enzyme that would uniformly speed along the small alkanes reaction to hydroxyl groups needed to produce fuel. (Image by CONG Zhiqi)

 中文版
中文版