Electrochemical CO2 reduction (CO2RR) is a promising method to convert CO2 into useful chemicals using renewable electric energy. It is one of the important ways to solve CO2 problem fundamentally. However, using inexpensive catalysts and obtaining both high faraday efficiency (FE) and high current density is a huge challenge. Manganese (MN) , as a transition metal with the third abundance in the Earth's crust, has shown great potential in many fields of electrocatalysis, but its performance in CO2RR is not satisfactory. In electrolyte, ionic liquid electrolyte can greatly reduce the reaction overpotential, which provides a special microenvironment for CO2RR. Recently, a new Mn-N3 single-atom catalyst for CO synthesis from CO2RR in ionic liquid electrolyte was reported by Zhang Suojiang research team of the Institute of Process Engineering, Chinese Academy of Sciences.
In this thesis, Mn single-atom catalysts were prepared on carbon nanotubes on the basis of carbon nitride. XPS showed that n atoms provided coordination sites for Mn atoms, and the distribution of Mn atoms on the surface of the catalyst could be observed by spherical aberration electron microscope, the coordination number of Mn-N is 3(Mn-N3) and the bond length is 2.21Å (figure 1) . The Mn-N4 structure of single atom catalyst is obviously different from the reported one. In KHCO3 electrolyte, the catalyst showed 98.8% CO FE and 14.0 mA cm-2 CO current density at 0.44 V overpotential, which was superior to the Mn-based catalysts reported. When ionic liquid was used as electrolyte, the CO current density was 29.7 mA cm-2.
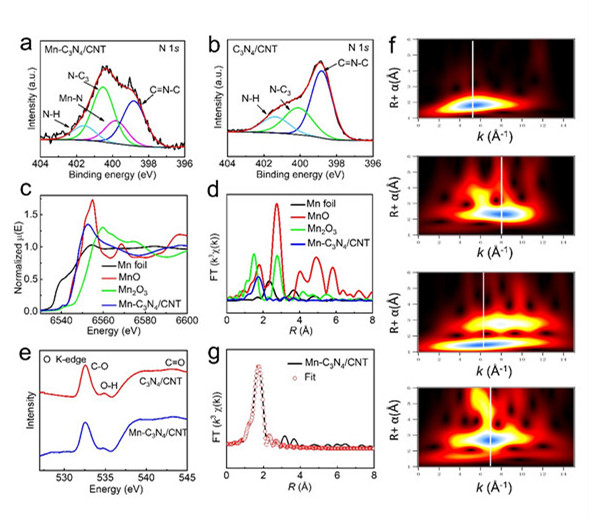
Fig. 1 structure characterization of catalyst
In this paper, in-situ characterization and simulation are used to study the mechanism of this process. In-situ XAFS shows that the valence state of Mn atom and the length of Mn-N bond are changed during the reaction, and CO2 is adsorbed on it, and electrons are converted to CO, which proves that the active site of the catalyst is Mn atom. DFT calculations show that the d-band center of Mn-N3 is closer to the FERMI energy level than that of Mn-N4, and the g of the critical intermediate on Mn-N3 is smaller and easier to occur (Fig. 2) .

Fig. 2 in situ characterization and mechanism analysis
In this paper, it is proved that the manganese-based catalyst with low activity can improve the activity of CO2RR by changing the structure of the active site, which provides an important scientific basis and feasibility for the electrocatalytic reduction of CO2 with low cost and high efficiency. At the same time, the strategy can be extended to other fields of electrocatalysis.
The research was accepted for publication by the International Journal Nature Communications.
(Jiaqi Feng, Hongshuai Gao, Lirong Zheng, Zhipeng Chen, Shaojuan Zeng, Chongyang Jiang, Haifeng Dong, Licheng Liu, Suojiang Zhang* and Xiangping Zhang*, A Mn-N3 single-atom catalystem bedded in graphitic carbon nitride for efficient CO2 electroreduction, Nature Communications, DOI: 10.1038/s41467-020-18143-y)

 中文版
中文版