Pyrazoline derivatives are a class of five-membered heterocyclic compound consisting of two connected n atoms and three C atoms. Because of its (C = N-NH-C) functional group, it has important applications in the fields of medicine, fluorescence and high energy fuel. In the field of medicine, N atom and C = N Double Bond on Pyrazoline ring can form p-conjugation, which can easily interact with various enzymes in organism, and have a wide range of biological activities. In the field of fluorescence, the p-conjugate effect of the pyrazoline ring makes the electrons in the ring delocalize and emit blue fluorescence, which can be used as fluorescent materials and fluorescent probes. In the field of high energy fuel, (C = N-NH-C) functional group can easily remove nitrogen to form a three-dimensional tension ring, which can be used as high energy tension ring fuel. Closed-loop synthesis of pyrazoline derivatives from keto-azobenzene is an effective method for the preparation of pyrazoline derivatives, which has the advantages of high atom utilization and good selectivity. At present, the main catalysts used for the synthesis of pyrazoline derivatives from keto-azobenzene are iodine or oxalic acid, although the yields of pyrazoline derivatives are high, they are homogeneous reactions, there are some disadvantages, such as difficult separation, heavy environmental pollution and complicated post-treatment.
For the first time, the ionic liquid research team at the Chinese Academy of Sciences has developed a method for preparing pyrazoline derivatives using green, heterogeneous FeCl3 catalysts that catalyze ketoazotization, it is helpful to solve the problems of catalyst separation and post-treatment. In this paper, the synthesis of pyrazoline derivatives catalyzed by FeCl3 was studied in detail by DFT calculations and experiments. In the aspect of calculation, the reaction path and reaction energy barrier (Fig. 1) for the synthesis of pyrazoline derivatives by ketohydrazine catalyzed by FeCl3 were calculated by DFT, the whole pathway can be divided into two basic reactions: keto-azobenzene configuration transition step and keto-azobenzene cyclization step. Compared with the reaction energy barrier without catalyst, FeCl3 can obviously reduce the reaction energy barrier of elementary reaction. Then the mode of action of FeCl3 catalyst and reaction substrate was calculated by electrostatic potential (ESP) . The results show that the addition of FeCl3 has a significant effect on the surface ESP distribution of Ketolidine (Fig. 2) . Before the addition of FeCl3, the minimum values of ESP on the surface of n 1 and n 2 atoms were similar (N 1 =-33.80, N 2 =-33.81 kcal/mol) . The addition of FeCl3 has a great effect on the maximum of ESP of H14 atom (3.22→21.72 kcal/mol) , which makes it easy to combine with N1, promote the transition of keto-diazonium configuration and initiate the cyclization reaction. The effects of reaction time, reaction temperature and catalyst dosage on the yield of pyrazoline derivatives were optimized experimentally (Fig. 3) . The experimental results show that under the optimal conditions, the conversion of keto-azobenzene reaches 95% and the selectivity remains 99% .
In this work, the method of preparing pyrazoline derivatives by ketoazotization catalyzed by FeCl3 was proposed, and the reaction path and energy barrier of transition state were calculated by DFT, which provided a new idea for the development of ketoazotization catalysts for pyrazoline derivatives.
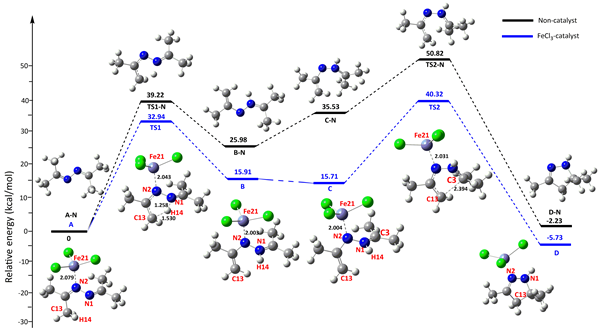
Figure 1. Reaction Pathway and transition state energy barrier in the synthesis of pyrazoline derivatives from keto-azobenzene
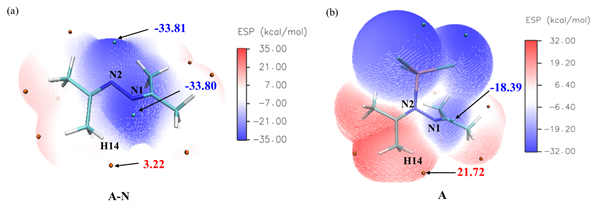
Fig. 2. Analysis of Ketoazonium surface electrostatic potential (ESP)
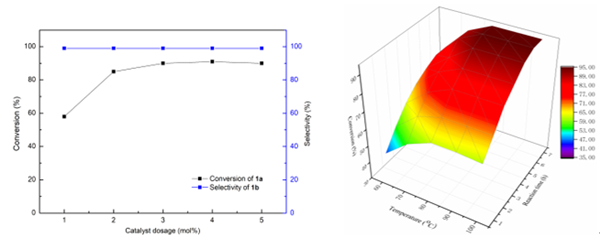
Figure 3. Effects of catalyst dosage, reaction time and reaction temperature on the selectivity of keto-azobenzene conversion
(Yangfeng Xia, Xing Zhang, Long Liu, Haiyun Sun*, Guoying Zhao and Yanqiang Zhang*,Highly efficient conversion of ketazines to pyrazoline derivatives catalyzed by FeCl3,Industrial & Engineering Chemistry Research,DOI: 10.1021/acs.iecr.0c04032)

 中文版
中文版